Structure of the ATP-driven methyl-coenzyme M reductase activation complex
Nature, Published online: 16 April 2025; doi:10.1038/s41586-025-08890-7The structure and function of the MCR activation complex from Methanococcus maripaludis were revealed, demonstrating its ATP-dependent ability to activate MCR and form methane while uncovering a unique electron transfer pathway involving iron–sulfur clusters similar to the nitrogenase cofactor intermediates.
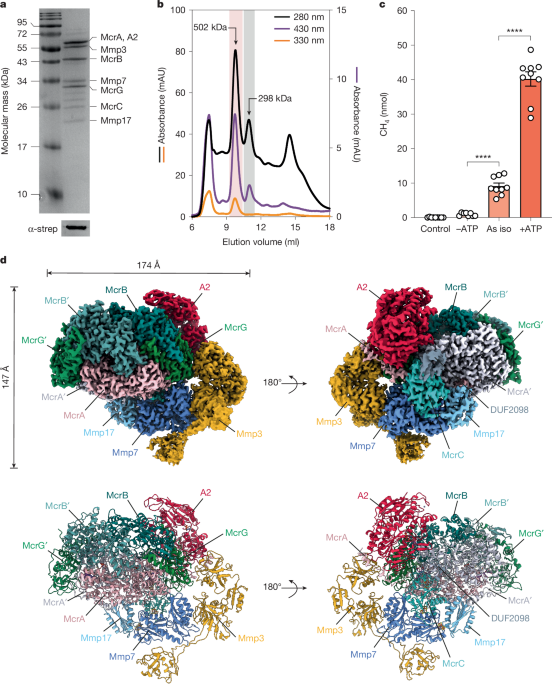
Nature, Published online: 16 April 2025; doi:10.1038/s41586-025-08890-7The structure and function of the MCR activation complex from Methanococcus maripaludis were revealed, demonstrating its ATP-dependent ability to activate MCR and form methane while uncovering a unique electron transfer pathway involving iron–sulfur clusters similar to the nitrogenase cofactor intermediates.