FDA Issues Serious Warning About Popular Hair Loss Drug
Be on the lookout.
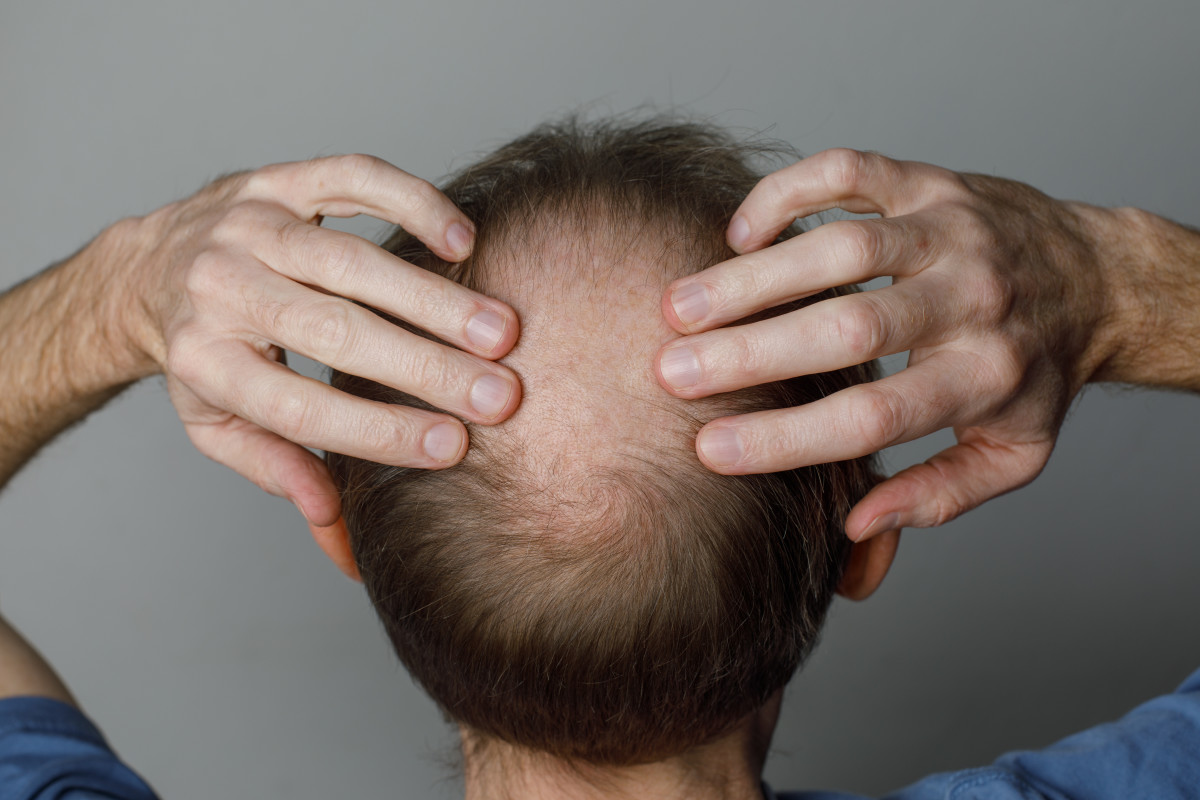
There's a widely-used hair loss drug that's causing alarm at the Food and Drug Administration, and now the federal agency is warning consumers.
The FDA says it has become aware of reports of adverse events involving compounded topical finasteride products potentially putting consumers at risk. The agency also says it is aware of some compounders and telemedicine platforms that market topical formulations of finasteride either as a single active ingredient (finasteride alone) or in combination with other active ingredients, such as finasteride combined with minoxidil, to treat hair loss.
Currently, there are only two FDA-approved oral finasteride products for different indications currently available in the U.S., and that includes Proscar (approved in June 1992) and Propecia (approved in December 1997).
But as far as topical formulation of finasteride, there is no product on the market that is FDA-approved. There are also no compounded topical finasteride products that are FDA-approved. And for those using those products, there are serious risks associated with its use.
According to the FDA, there are 32 reported cases of adverse events between 2019 and 2024. Some adverse effects includes absorption of finasteride through the skin into the bloodstream. Other adverse side effects involved with topical finasteride products include erectile dysfunction, anxiety, suicidal ideation, brain fog, depression, fatigue, insomnia, decreased libido and testicular pain.
Related: Are Hair-Loss Drugs Safe?
The agency also warns that adverse events continued to persist after product discontinuation.
"In addition to safety concerns described in the labeling of FDA-approved oral finasteride products, topical finasteride poses other risks such as local reactions including irritation, erythema, dryness/scaling, stinging and burning, and greater potential risk for inadvertent exposure to others, specifically females, through transfer of applied product," the agency states.
According the The Wall Street Journal, a 26-year-old U.S. Army sergeant's side effects included anxiety, dizziness, and slurred speech. Even worse, his sex drive severely declined and his genitals shrank and changed shape. According to the outlet, none of the men who spoke to them say they were warned about the side effects.
The FDA now implores health care providers to educate patients on the potential risks.












![[DEALS] Sterling Stock Picker: Lifetime Subscription (85% off) & Other Deals Up To 98% Off – Offers End Soon!](https://www.javacodegeeks.com/wp-content/uploads/2012/12/jcg-logo.jpg)